Mechanism of Soft Tissue Injury and Repair Phases of Healing
Personal Injury Institute, Personal Injury Report
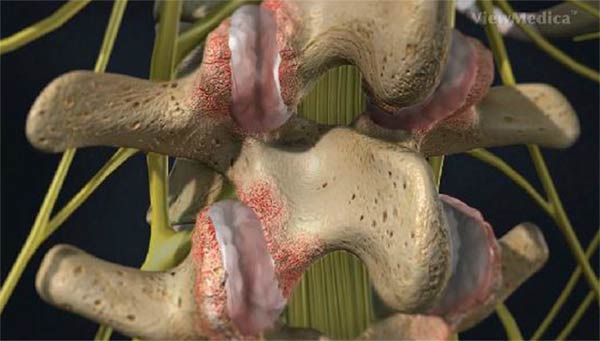
The term “whiplash” is a colloquialism. “Cervical acceleration–deceleration” (CAD) describes the mechanism of the injury, while the term “whiplash associated disorders” (WAD) describes the injury sequelae and symptoms.
Whiplash is commonly associated with motor vehicle accidents, usually when the vehicle has been hit in the rear;[3] however, the injury can be sustained in many other ways, including headbanging,[4] bungee jumping and falls.[5] It is one of the main injuries covered by insurance.[citation needed] In the United Kingdom, 430,000 people made an insurance claim for whiplash in 2007, accounting for 14% of every driver’s premium.[6]
Before the invention of the car, whiplash injuries were called “railway spine” as they were noted mostly in connection with train collisions. The first case of severe neck pain arising from a train collision was documented around 1919.[7] The number of whiplash injuries has since risen sharply due to rear-end motor vehicle collisions. Given the wide variety of symptoms associated with whiplash injuries, the Quebec Task Force on Whiplash-Associated Disorders coined the phrase ‘Whiplash-Associated Disorders’.[7]
Whiplash can be described as a sudden strain to the muscles, bones and nerves in the neck. The neck is made up of seven vertebrae, referred to as the cervical vertebrae. The first two cervical vertebrae, the axis and atlas, are shaped differently from the remaining five. The atlas and axis are responsible for movement of the skull from side to side (cervical rotation to the right and left); also moving forward and backward (cervical flexion and extension). Excessive extension and flexion can disrupt the vertebrae.
 There are four phases that occur during “whiplash”: Initial position (before the collision), retraction, extension and rebound. In the initial position there is no force on the neck it is stable due to inertia.[22] Anterior longitudinal ligament injuries in whiplash may lead to cervical instability.[23] They explain that during the retraction phase that is when the actual “whiplash” occurs, since there is an unusual loading of soft tissues. The next phase is the extension, the whole neck and head switches to extension, and it is stopped or limited by the head restraint. The rebound phase transpires as result of the phases that are mentioned.
There are four phases that occur during “whiplash”: Initial position (before the collision), retraction, extension and rebound. In the initial position there is no force on the neck it is stable due to inertia.[22] Anterior longitudinal ligament injuries in whiplash may lead to cervical instability.[23] They explain that during the retraction phase that is when the actual “whiplash” occurs, since there is an unusual loading of soft tissues. The next phase is the extension, the whole neck and head switches to extension, and it is stopped or limited by the head restraint. The rebound phase transpires as result of the phases that are mentioned.
During the retraction phase the spine forms an S-Shaped curve, and this caused by the flexion in the upper planes and hyperextension at the lower planes and this exceed their physiological limits this phase the injuries occur to the lower cervical vertebrae. At the extension phase all cervical vertebrae and the head are fully extended, but do not surpass their physiological limits. Most of the injuries happen in C-5 and C-6.[24]
While the time associated with a specific collision will vary, the following provides an example of the occupant and seat interaction sequence for a collision lasting approximately 300 milliseconds.
Mechanism of Soft Tissue Injury
One of the most injurious forces to neck–one that is rarely seen elsewhere and one that nature probably never intended humans to endure–is horizontal shear force, which is not seen unless someone was in a rear impact collision. As noted earlier, this is accompanied by cervical compression, which can also be injurious. This occurs chiefly within the first 100-125 msec, and generally prior to the time the head makes contact with the head restraint.
In some cases, as a result of ramping, the height of the occupant, or a head restraint that’s adjusted in the down position, the head may extend over the head restraint and, given sufficient impact velocity, hyperextension may occur. In most cases, however, injury occurs before this point and hyperextension is not a prerequisite for injury.
 Also as mentioned in an earlier section, stretching of joint capsules, vertebral arteries, and intersegmental hyperextension at lower cervical levels can result in long-term pain and biomechanical compromise as a result of ligamentous subfailure, disc disruption, etc. Depending on the occupant’s kinematics and the nature of the crash, forward kinematics can be just as potentially injurious as rearward kinematics. For example, when the occupant’s struck vehicle then collides with the vehicle in front of it–the so-called second collision–the injury kinematics may be compounded with a flexion bending moment superimposed upon the extension moment.
Also as mentioned in an earlier section, stretching of joint capsules, vertebral arteries, and intersegmental hyperextension at lower cervical levels can result in long-term pain and biomechanical compromise as a result of ligamentous subfailure, disc disruption, etc. Depending on the occupant’s kinematics and the nature of the crash, forward kinematics can be just as potentially injurious as rearward kinematics. For example, when the occupant’s struck vehicle then collides with the vehicle in front of it–the so-called second collision–the injury kinematics may be compounded with a flexion bending moment superimposed upon the extension moment.
Naturally, muscles can also be injured, and the nerve roots and brachial plexus can be stretched (1); the brain, brain stem, and spinal cord can also be injured. Disc separations from vertebrae (rim lesions), along with small fractures, significant hemarthroses, and synovial injuries to facet joints, have been observed at autopsy in persons who survived their injuries, but died later of other causes (2, 3, 4). In many cases the lesions seen were not visible on radiographs, even in retrospect.
Recently, an intriguing new hypothesis for injury has been tested by Svensson et al. (5, 6). They demonstrated CSF pressure gradients in pig spines subjected to extension motions with angular displacements and durations equivalent to those seen in typical CAD trauma. They found pressure pulses 10 times higher than normal. These pulses produce pressure gradient injuries to cervical and upper thoracic dorsal root ganglia which were demonstrable on light microscope examination and which are postulated as a mechanism of nerve injury.
Finally, because so many of the symptoms and conditions, like TOS, TMD, PCS, CTS, etc., develop subsequent to CAD injury–some developing weeks or months later–CAD syndrome should be looked upon as a process rather than a single event.
There are many published journal articles and books pertaining to the topic of “Soft Tissue Injury and Repair.”
- Studies on development of connective tissue in transparent chambers in rabbit’s ear; American Journal of Anatomy; 1940.
- Orthopaedic Medicine, Diagnosis of Soft Tissue Lesions; 1982.
- Acute soft tissue injuries; Australian Family Physician, 1982.
- Sports Medicine: Prevention, Evaluation, Management, and Rehabilitation; 1983.
- Normal ligament Properties and Ligament Healing; Clinical Orthopedics and Related Research; 1985.
- Acute soft tissue injuries-a review of the literature; Medicine and Science of Sports and Exercise; 1986
- Injury and Repair of the Musculoskeletal Soft Tissues; American Academy of Orthopaedic Surgeons; 1988.
- Wound Healing, Biochemical & Clinical Aspects; 1992.
- Continuous Passive Motion, A Biological Concept for the Healing and Regeneration of Articular Cartilage, Ligaments, and Tendons; From Origination to Research to Clinical Applications; 1993.
- Effects of Early Motion on Healing of Musculoskeletal Tissues, Hand Clinics; 1996.
- Scar Formation and Ligament Healing; Canadian Journal of Surgery; 1998
- Immobilization or Early Mobilization After an Acute Soft-Tissue Injury?; The Physician And Sports Medicine; 2000.
- Cells, Tissues, and Disease: Principles of General Pathology, 2004.
- The Basics of Soft Tissue Healing and General Factors that Influence Such Healing; Sports Medicine Arthroscopic Review; 2005.
- Orthopedic Biology and Medicine; Repair and Regeneration of Ligaments, Tendons, and Joint Capsule; Humana Press, 2006.
- Fascia: The Tensional Network of the Human Body; The Scientific and Clinical Applications in Manual and Movement Therapy; 2012.
Fascia
The Tensional Network of the Human Body
The Scientific and Clinical Applications in Manual and Movement Therapy
In this book, they add to the details of the principles of soft tissue injury and repair. Specifically, they state: “Wound healing is divided into three or four phases:”
Phase 1A: The Vascular Inflammatory Phase
This phase begins immediately after injury and last 0 – 2 days. It is characterized by bleeding into the tissue.
Phase 1B: The Cellular Inflammatory Phase
Fibroblasts migrate into the injury area. This phase lasts 2 – 5 days.
Phase 2: The Proliferation Phase
This phase begins at the fifth day and lasts about 21 – 28 days. “The proliferation phase in poorly perfused tissue such as tendon, ligament, meniscus, or intervertebral disc can last up to 6 weeks.”
In this phase, wound closure occurs with a network of collagen fibers. “For this collagen network to attain an almost identical construction of the original tissue, the tissue in this phase of would healing must be confronted with its normal physiological stress.”
Phase 3: The Consolidation Reconstruction Phase
This phase begins between days 21 – 28, and continues to about day sixty.
Phase 4: The Remodeling / Maturation Reconstruction Phase
This phase begins at about day 60 and continues for about a year. “Stress on the tissue should now be slowly increased to push ahead the reconstruction of unstable type III collagen to stable type I collagen and return the tissue to its original stability.”
Improvements in Timing and Quality of Healing
There are a number of variables that influence the timing and quality of healing.
Dr. Kellett notes (6):
“Cryotherapy (crushed ice) for 10 to 20 min, 2 to 4 times/day for the first 2 to 3 days is helpful in promoting early return to full activity.” “Numerous studies have shown the effectiveness of ice therapy in reducing the pain and period of disability to soft tissue injuries.” In contrast, “early heat treatment leads to an increase in the blood flow to the injured area with an exaggerated acute inflammatory response.”
“The advantages of cryotherapy in treating soft tissue injuries have been well documented.” Ice within 48 hours of injury reduces disability of ankle sprains from 15 days to 10 days.
“Early mobilization, guided by the pain response, promotes a more rapid return to full activity.”
“Early mobilization, guided by the pain response, promotes a more rapid return to full functional recovery.”
“Progressive resistance exercises (isotonic, isokinetic, and isometric) are essential to restore full muscle and joint function.”
 Following this acute inflammatory phase and largely guided by the pain response of the patient, early mobilization is commenced, based upon the premise that the stress of movement on repairing collagen is largely responsible for the orientation and tensile strength of the tendons and ligaments.
Following this acute inflammatory phase and largely guided by the pain response of the patient, early mobilization is commenced, based upon the premise that the stress of movement on repairing collagen is largely responsible for the orientation and tensile strength of the tendons and ligaments.
The goal of stressing repairing tissues with controlled motion is to induce adaptive response of functionally stronger connective tissues. However, excessive stressing of the repairing tissues may result in further damage. Consequently, any sign or symptom which suggests a worsening of the injury (severe pain) is a clear indication to reduce the motion stress on the tissues.
The strength of repaired ligaments is proportional to the mobility of the ligament, resulting in larger diameter collagen fiber bundles and more total collagen. “Collagen fiber growth and realignment can be stimulated by early tensile loading of muscle, tendon, and ligament.”
•••
In 1998, Drs. Hildebrand and Frank note (11):
“Motion in stable joints improves the biomechanical properties of healing ligaments compared with immobilization of joints.” “The mechanism presumably involves the application of controlled forces; too little or too much force is detrimental.”
•••
In 2005, Dr. Hildebrand and colleagues note (14):
“The large scar tissue mass gradually remodels, likely under the influence of the mechanical environment.”
“Maturation of the scar tissue requires mechanical loading to continue the remodeling phase of healing.”
“Normal connective tissues that function in a mechanically active environment (actually most tissues) subscribe to the ‘‘use it or lose it’’ paradigm of tissue integrity.” “Increased loading leads to adaptation, whereas decreased loading below a threshold leads to atrophy.” “The same principle likely also holds for scar tissue and immobilization beyond the initial phases of healing could have a detrimental impact on outcome.”
“Mechanobiology is likely important in the healing outcome in tissues such as ligaments, tendons, and related tissues. That is, depriving healing ligaments of mechanical loading likely has a detrimental impact on healing outcome.”
•••
Dr. Schleip and colleagues note (16):
“For this collagen network to attain an almost identical construction of the original tissue, the tissue in this phase of would healing must be confronted with its normal physiological stress.”
“Stress on the tissue should now be slowly increased to push ahead the reconstruction of unstable type III collagen to stable type I collagen and return the tissue to its original stability.”
“Because connective tissue consists predominantly of proteins, the take-up of protein through nutrition is very important.”
“Matrix and cells are found in a continuous dialogue and are dependent on each other.” “The forces on the network of collagen and elastic fibers and ground substance are transferred to the cell membranes via link proteins. The cell is informed by these signals and is stimulated to keep synthesizing new matrix components. This rebalances the physiological breakdown of the matrix and the tissue retains its stability and mobil-ity. A reduction in the load stimulus leads as a result to reduced synthesis activity of the cells and thus to a loss of matrix components.”
“This produces a lower level of stability and limited mobility because of the formation of pathological cross links. An important task for the therapist is to apply gradually increasing levels of force without causing pain, in order to promote the healing and regeneration processes and in this way restore mobility and stability.”
•••
Problems and Residual Characteristics
Most healed soft tissue injuries are asymptomatic. However, it is universally accepted that the healed tissue is weaker than the pre-injured tissue. Consequently, acute flare-ups of pain or exacerbations of pain and/or spasm often occur as a consequence of increased use or stress of the once injured but now healed tissues. Good early treatment improves the quality and timing of soft tissue injury. Best early treatment appears to include ice and early controlled motion that does not move the tissues into pain.
The chronic tissue residuals following trauma and healing are histologically provable. Common terminology to describe them includes “scar, fibrosis, fibrosis of repair, adhesions,” etc. A collection of these principles follows:
Dr. Kellett notes (6):
“The micropathology of acute soft tissue trauma has been investigated. Healing of ligaments and soft tissue injuries in general has been shown to occur by fibrous repair (scar tissue) and not by regeneration of the damaged tissue.”
“Normal ligaments are composed of type I collagen, whereas damaged (and healed) ligaments contain a large proportion of immature type III collagen which is deficient in the number of cross-linkages between and within the tropocollagen subunits.”
The remodeled scar is deficient in both content and quality 40 weeks after injury, as there is a plateau in scar collagen concentration at about 70% of normal.
•••
In 1998, Drs. Hildebrand ad Frank note (11):
“Injuries to ligaments induce a healing response that is characterized by the formation of a scar.” This “scar tissue is weaker.”
“Ligament healing is characterized by the formation and remodeling of scar tissue that is weaker than normal ligament owing to alterations in biochemical composition and structural organization.” “The scars have a greater amount of inferior strength tissue compared with that of normal” ligaments.
“The structural strength and stiffness, stress and tissue quality continue to improve up to 12 months after injury, but after that time only relatively small increases are made. However, the material properties of the ligament scar do not return to normal even after 2 years.”
Residual scar tissue behaves with “abnormal biomechanical, biochemical and ultrastructural properties.”
“The return of joint function after injury does not mean that the ligament has healed.”
“Ligament healing in what may be considered to be the best case scenario is characterized by a scar material with inferior tissue quality, with changes in biochemical and histologic properties, that does not regenerate a normal ligament even after 2 years of healing.”
The ligament scar affects the associated joint function.
•••
In 2005, Dr. Hildebrand and colleagues note (14):
“Whereas wound healing generally leads to a repair of the injured site, it does not lead to tissue regeneration. This difference between repair and regeneration has influence on tissues such as ligaments and tendons that function in a mechanically active environment.”
The repair process can lead to a loss of function, primarily from scar tissue, and this can occur in both musculoskeletal and visceral tissue (heart, lung, kidney, liver).
“The remodeling phase is a slow process and is accompanied by alterations not only in matrix remodeling, but also gene expression, cellularity, vascularity, and innervation.” “Because the remodeling phase occurs slowly, and may take months (i.e., skin) or years (i.e., tendon and ligament).”
“Even after protracted time post-injury, the mechanical properties of a scar tissue in a ligament … is still compromised compared with normal.”
“The scar tissue may be functional for most activities even though it is not ‘normal’.” [Fails during high demand activities]
The scar cells in the healing ligament are different from normal cells and therefore the scar is intrinsically different.
‘‘Scar-like’’ tissue is “functionally ineffective.”
“Some tendon and ligament injuries lead to formation of scar tissue that is partially functional, but to regain as much function as possible requires physiotherapy to ‘facilitate’ the return to function after the scar tissue has formed.”
The inflammatory response associated with overt injury or surgery can lead to formation of adhesions, where “the ligament/tendon scar tissue is ‘‘bonded’’ to the surrounding tissue and thus, such restrictions compromise function in situations where movement is required.”
“The size of the wound and the resulting scar tissue has a dramatic impact on the biomechanical outcome.”
“It is readily apparent that wound healing in the adult under the most optimal conditions should be considered tissue repair not regeneration.” “For tissues like a ligament or tendon, the mechanical outcome may be less than ideal, depending on the expectations of tissue use post-injury and the occurrence of side-effects such as adhesions.”
Additionally, Dr. Hildebrand and colleagues discuss factors that impair soft tissue healing. These factors cause some patients to heal slowly or incompletely with more functional residuals. These factors include:
- Older age
- Female Sex
- Genetics
- Tissue history (prior injuries, scar tissue, and disease states)
- Diabetes
- Disc injuries heal poorly, primarily as a consequence of poor blood supply
- Pregnancy
- Low Vitamin C levels
- Menstrual cycle hormonal changes
- Any reason that deprives the healing tissues from mechanical loading
- Re-injury of a prior injury or prior tissue that has sustained repetitive stress
- Excess carbohydrates that increase glycation (AGEs)
- Any treatment with corticosteroids
- Anything that exaggerates or prolongs the inflammatory response
•••
Dr. William Walsh and colleagues note (15):
“Musculoskeletal soft tissues tend to heal through formation of relatively weak, disorganized scar tissue. Severe ligament sprains can be particularly problematic; even in the best case, there is a fibrotic repair response that remodels over several years, causing scar tissue that is structurally and biomechanically inferior to the normal tissue. For most soft tissues, the fibrotic repair process is thought to interfere with the possibility of regeneration.” “Soft tissues typically heal by fibrosis, potentially impairing regeneration.”
“Ligaments take longer to heal than other connective soft tissues, and the repaired ligament tissue is scarlike and inferior to normal ligament tissue both biologically and biomechanically. Furthermore, some ligament-deficient joints subsequently become unstable and can lead to lifelong disability with osteoarthritis.”
“Ligament wounds, even over 2 yr after injury, contain mainly a homogenous population of small-collagen fibrils as commonly observed in scar tissue, with a few patches of normal larger fibrils being observed. These ligaments never obtain their original biomechanical properties.”
These concepts on ligament repair have important applications for chiropractors that treat spinal trauma patients:
Soft tissue healing occurs over a period of 12 months or more. Controlled motion, including the chiropractic adjustment, is the best approach to enhance the quality of ligament healing, especially in the remodeling phase.
Ligaments and tendons heal with scar tissue (repair) as a rule, and not with normal pre-injury tissue (regeneration). This scar tissue causes permanent loss of function. Even healed ligament injuries have residual weakness.
Scar tissue is mechanically functionally inferior to normal tissue. Its inherent weakness makes the tissue prone to failure at previously normal load levels, and subsequent new trauma to scarred tissue will result in greater injury.
Scar tissue (fibrosis) is linked to the intensity of the initial inflammatory response. Consequently, early inflammation control (ice) could improve the timing and quality of healing. Scar tissue, to varying degrees, is remodelable with the application of controlled motion, which I believe includes and even requires chiropractic adjustments.
Lack of symptoms is not synonymous with full healing and functional recovery. A flare-up or exacerbation of pain after the healing process most likely will require another few quick visits to the chiropractor.
•••
SUMMARY:
This review reminds us that it is well established that:
- Whiplash injury chronic pain is primarily generated by injury to the facet joint capsular ligaments.
- Facet joint capsular chronic pain can cause an abnormal psychological profile.
- The abnormal psychological profile caused by chronic facet pain can only be successfully treated if the chronic pain is successfully treated.
- Many studies conclude that litigation (hiring a lawyer) subsequent to an injury is “harmful to recovery.” However, these studies do not evaluate the concept of Reverse Causality, and hence are flawed.
- When Reverse Causality is carefully evaluated, litigation not only does not harm recovery, data suggests it actually improves recovery.
•••
References:
1. Insurance Institute for Highway Safety. “Q&A: Neck Injury”. Retrieved 2007-09-18. “whiplash” at Dorland’s Medical Dictionary
2. Krafft, M; Kullgren A; Lie A; Tingval C (2005-04-01). “Assessment of Whiplash Protection in Rear Impacts” (pdf). Swedish National Road Administration & Folksam. Retrieved 2016-01-10. http://www.nhs.uk/conditions/whiplash/Pages/Introduction.aspx
3. (2010). Retrieved January 16, 2013 from http://www.njpcc.com/conditions-of-the-spine/neck-paininjury.html
4. “Warning over whiplash ‘epidemic'”. BBC News. 2008-11-15. Retrieved 2010-04-06.
5. Desapriya, Ediriweera (2010). Head restraints and whiplash : the past, present, and future. New York: Nova Science Publishers. ISBN 978-1-61668-150-0.
6. Bismil QM, Bismil MS (2012). “Myofascial-entheseal dysfunction in chronic whiplash injury”. J R Soc Med Sh Rep. 3 (8): 57. doi:10.1258/shorts.2012.012052.
7. Gorski JM and Schwartz LH, Shoulder Impingement Presenting as Neck Pain. The Journal of Bone & Joint Surgery Vol 85-A • Number 4 • April 2003 p635-638
8. Chauhan SK, Peckham T, Turner R (2003). “Impingement Syndrome Associated with Whiplash Injury”. J Bone Joint Surg. 3: 408–410.
9. Umaar W. Yew KIM; Zenios M.; Brett I.; Sharma Y. (2005). “Whiplash Injury of the Shoulder: Is it a Distinct Clinical Entity?”. Acta Orthop. Belg. 71: 385–387.
10. Abbassian A.; Giddins G. (2008). “Subacromial Impingement in Patients with Whiplash Injury to the Cervical Spine”. Journal of Orthopaedic Surgery and Research. 3 (25): 1749.
11. Borenstein, P.; Rosenfeld, M.; Gunnarsson, R. (2010). “Cognitive symptoms, cervical range of motion and pain as prognostic factors after whiplash trauma.”. Acta Neurol Scand. 122 (4): 278–85. doi:10.1111/j.1600-0404.2009.01305.x. PMID 20003080. MedlinePlus Encyclopedia Whiplash
12. Roller Coaster Neck Pain, from the Spinal Injury Foundation
13. “Whiplash injury”. 2006-08-23.
14. “shaken baby syndrome”. healthily.com. Retrieved 2015-07-22.
15. Castro, W. H.; Meyer, S. J.; Becke, M. E.; Nentwig, C. G.; Hein, M. F.; Ercan, B. I.; Thomann, S.; Wessels, U.; Du Chesne, A. E. (2001). “No stress–no whiplash? Prevalence of “whiplash” symptoms following exposure to a placebo rear-end collision”. International journal of legal medicine. 114 (6): 316–322. doi:10.1007/s004140000193. PMID 11508796.
16. Patijn J, Wilmink J, ter Linden FHJ, Kingma H. CT study of craniovertebral rotation in whiplash injury. European Spine J 10:38-43, 2001.
17. Jónsson H Jr, Bring G, Rauschning W, Sahlstedt B: Hidden cervical spine injuries in traffic accident victims with skull fractures. J Spinal Dis 4:251-263, 1991.
18. Taylor JR, Twomey LT: Acute injuries to cervical joints. Spine 18(9):1115-1122, 1993.
19. Taylor JR, Kakulas BA: Neck injuries. Lancet 338:1343, 1991.
20. Svensson MY, Aldman B, Hansson HA, et al.: Pressure effects in the spinal canal during whiplash extension motion: a possible cause of injury to the cervical spinal ganglia. International IRCOBI Conference on the Biomechanics of Impact, Eindhoven, Netherlands,189-200, 1993.
21. Örtengren T, Hansson H-A, Lövsund P, Svensson MY, Suneson A, Säljö A: Membrane leakage in spinal ganglion nerve cells induced by experimental whiplash extension motion: a study in pigs. J Neurotrauma 13(3):171-179, 1996.
22. Kandel ER, Schwartz JH, Jessell TM; Principles of Neural Science, Elsevier; 2000.
23. Bogduk N; On Cervical Zygapophysial Joint Pain After Whiplash; Spine December 1, 2011; Volume 36, Number 25S, pp. S194–S199.
24. Bogduk N, Aprill C; On the nature of neck pain, discography and cervical zygapophysial joint blocks; Pain; 54; 1993, pp. 213-217.
25. Wallis, BJ, Lord, SM and Bogduk, N (1997). “Resolution of psychological distress of whiplash patients following treatment by radiofrequency neurotomy: a randomized, double-blind, placebo-controlled trial.” Pain; 73: pp. 15-22.
26. Spearing NM, Connelly LB; Is compensation “bad for health”? A systematic meta-review; Injury; January 2011; Vol. 42; No. 1; pp. 15-24.
27. Scholten-Peeters GGM, Verhagen AP, Bekkering GE, van der Windt DAWM, Barnsley L, Oostendorp RAB, Hendriks EJM; Prognostic factors of whiplash-associated disorders: A systematic review of prospective cohort studies; Pain ; July 2003, Vol. 104, pp. 303–322.
28. Spearing NM, Connelly LB, Gargett S, Sterling M; Does injury compensation lead to worse health after whiplash? A systematic review; Pain; June 2012; Vol.153; No. 6; pp.1274-82.
29. Cassidy JD, Carroll LJ, Côté P, Lemstra M, Berglund A, Åke Nygren A; Effect of Eliminating Compensation for Pain and Suffering on the Outcome of Insurance Claims for Whiplash Injury; New England Journal of Medicine; April 20, 2000; Vol. 342; No. 16; pp. 1179-1186.
30. Spearing NM, Connelly LB, Nghiem HS, Pobereskin L; Journal of Clinical Epidemiology; November 2012; Vol. 65; No. 11; pp. 1219-1226.



